Outcomes of long-term maintenance infliximab therapy in adult patients with moderate to severe Crohn’s disease from March 2003 to December 2010
We evaluated the long-term clinical benefit of infliximab (IFX) in a total of 74 patients with moderate to severe Crohn’s disease (CD); the median follow-up was 16 months (interquartile range 13–35). Methods: We compared the quality of mucosal healing (MH) in 3 groups of patients with CD. Group I (GI): CD duration < 3 years, treated with IFX. Group II (GII): CD duration > 3 years, treated with IFX. Group III (GIII): CD duration > 3 years, treated with IFX + azathioprine (AZA). Results: At week 52, deep clinical remission was observed in seven of 21 patients in GI (33.33%), in three of 19 patients in GII (15.70%), and in eight of 20 patients in GIII (40.00%). The differences among groups were statistically significant in the likelihood ratio test (P = 0.019) and log- -rank test (P = 0.042). The covariate analysis showed significant differences in the outcomes between GIII and GII (OR 7.20, P = 0.05) and between GI and GII (OR 6.3, P = 0.052), and a nonsignificant difference between GIII and GI (OR 1.143, P = 0.975). Sustained long-term clinical benefit was observed in 47 (63.9%) of the initial responders. Conclusion: IFX and AZA combination therapy was highly effective in patients with CD duration > 3 years after failure of conventional therapy. Timely IFX monotherapy works best in patients with evidence of active inflammation as demonstrated by an elevated C-reactive protein and in patients with nonstricturing disease.
Introduction
Crohn’s disease (CD) is a chronic inflammatory disease of the gastrointestinal tract which is characterized by recurrent inflammation followed by periods of remission and inactive disease. Over time, it progresses to complications such as strictures, fistulae, and abscesses [1]. Infliximab (IFX) is a mouse chimeric immunoglobulin G1 (IgG1) monoclonal antibody to tumor necrosis factor alpha (TNF-alpha). IFX not only maintains disease improvement, but also induces mucosal healing in nearly half of the treated patients [2, 3].
Aims and Methods
The study compared the quality of mucosal healing (MH) in moderate to severe Crohn’s disease during a long-term maintenance therapy with infliximab in three groups of patients from March 2003 to December 2010.Group I (GI): n = 22 patients (30%), duration of Crohn’s disease < 3 years, treated with IFX.
Group II (GII): n = 29 (39%), duration of Crohn’s disease > 3 years, treated with IFX.
Group III (GIII): n = 23 (31%), duration of Crohn’s disease > 3 years, treated with IFX and azathioprine.
Primary end-points: Clinical disease activity was scored according to the Crohn’s disease Activity Index (CDAI). Clinical remission was defined as an absolute CDAI score of less than 150 points at least for three months [4]. A CDAI score of less 150 points indicated clinically inactive disease [4]. Endoscopic disease activity was defined by the Simple endoscopic score for CD (SES-CD)
For practical reasons, we decided to give a numeric value to complete endoscopic remission – a SES-CD of < 3. The SES-CD range was from 0 to 30 points. SES-CD = Total – 1.4 × n (Total = Raw sum of variables; n = number of affected segments). To calculate the SES-CD was used calculator SES-CD [5]. SES-CD < 3 was defined as endoscopically inactive disease [5–7].
Complete mucosal healing (MH) was defined as the absence of ulcerations or erosions in any segment at follow-up endoscopy in patients who had one present at baseline ileocolonoscopy. Partial MH was defined as clear endoscopic improvement with no deep ulceration in any segment, SES-CD < 5.5. Deep clinical remission was defined as clinical remission with a CDAI score of less 150 points and a complete MH. Sustained clinical benefit was defined as a lasting control of the disease activity during follow-up with permanent improvement of the symptoms. Blood samples were collected for testing C-reactive protein (CRP normal value was from 0 to 6 mg/L) and the presence of antibodies to IFX between weeks 30 and 54.
Acute infusion reaction was defined as any adverse event (AE) that occurred during or within 1 hour after infusion of IFX. Any reaction seen later was classified as delayed. Serum sickness-like reaction was defined as a clinical symptom complex manifested as myalgia, fever, rash, or any combination of these symptoms. Severe adverse event (SAE) was defined as any AE which resulted in hospitalization, was fatal, or led to disability.
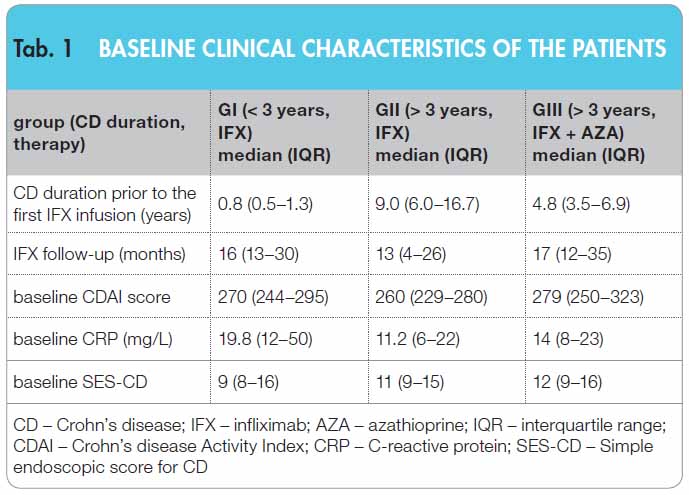 A total of 74 patients with moderate to severe CD were treated with IFX from March 2003 to December 2010. Median age at diagnosis was 29 years; interquartile range (IQR) 22–37, CDAI score range ? 220–450 points; for details, see Table 1.
A total of 74 patients with moderate to severe CD were treated with IFX from March 2003 to December 2010. Median age at diagnosis was 29 years; interquartile range (IQR) 22–37, CDAI score range ? 220–450 points; for details, see Table 1.
IFX was given at a dose of 5 mg/kg of body weight at weeks 0, 2, and 6 and then every 8 weeks. Oral azathioprine was administered at a dose of 1.5–2.5 mg/kg/day and systemic corticosteroid at a dose of less than 20 mg/day until week 14 and subsequently, the dose was reduced. The median intervals from colonoscopy to the first IFX infusion were 2 weeks (IQR 1.5–6) in GI, 2.1 (3.1–7.9) in GII, and 3.6 (3–5.2) in GIII.
Of 74 patients receiving infliximab therapy, 35 were females and 25 were non-smokers. At diagnosis, 64.8% of CD patients had ileocolic disease, 21.6 % ileitis and proximal small bowel disease, 13.5% colitis, and 47.3% perianal manifestations.
Altogether 73.7% of patients had luminal CD, 15.8% stricturing CD, and 10.8% penetrating CD. Seventy-three percent of patients were aged less than 40 years. Previous bowel surgery was performed in 42.1% of patients.
Statistical analysis: For all end-points, (a) the proportions of patients reaching a given end-point by week 52 were estimated and compared using the likelihood ratio (LR) test in a logistic regression with group (I, II, III) as a predictor and (b) the overall group-specific survival curves were compared using the log-rank test. Finally, the odds ratios were calculated to compare the groups. The 95% confidence intervals (CI) and P-values in this multiple comparison were adjusted using the methods of Hothorn [8].
Main results
Week 12
IFX was discontinued due to AEs in 10 patients (13.5%) of whom nine developed infusion reaction. Three patients (4.05%) were primary non-responders and one was non-compliant. After completion of induction phase, 60 (21 in GI, 19 in GII, and 20 in GIII) of the 74 patients continued on the scheduled therapy.
Week 52
Twenty-one of 22 patients in GI, 19/29 in GII, and 20/23 in GIII were in clinical remission. The rates of patients with a CDAI score of < 150 points were 95.5% in GI; 95% CI (63.5–93.5), 82.8% in GII; 95% CI (63.5–93.5), and 87.0% in GIII; 95% CI (65.3–96.6); P-value (week 52) = 0.338; log-rank P-value = 0.272.
All patients underwent endoscopic check-up between weeks 52 and 56 of follow-up. Mucosal healing was observed in 47 (63.9%) of the initial responders. Complete MH was achieved in 18 (23%) of 47 patients. Partial MH was in 29 (40.9%) of 47 patients.
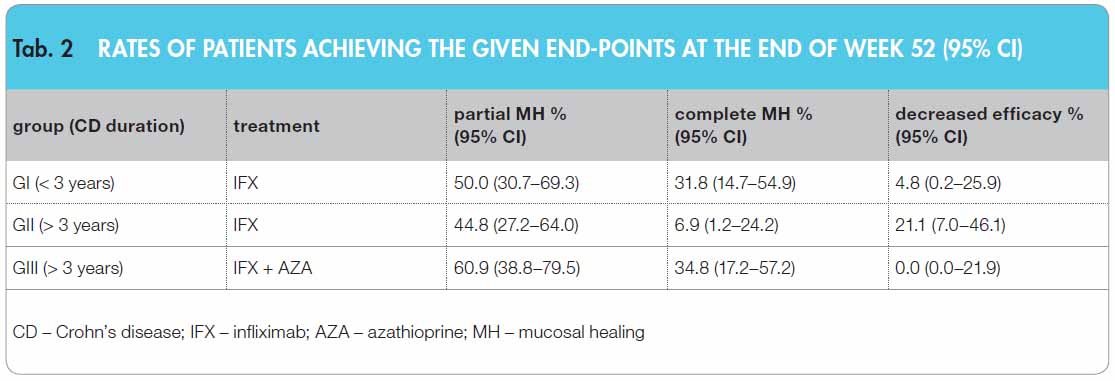 The largest proportions of patients with complete MH were in GIII: 8/20 and GI: 7/21, followed by GII initial responders: 3/19. The rates of patients reaching complete MH at 95% CI are given in Table 2.
The largest proportions of patients with complete MH were in GIII: 8/20 and GI: 7/21, followed by GII initial responders: 3/19. The rates of patients reaching complete MH at 95% CI are given in Table 2.
The differences in MH rates among groups I, II, and III were statistically significant at the end of week 52 (in the likelihood ratio test P = 0.019). When comparing the times required to achieve complete MH, we found significant differences among groups (in the log-rank test P = 0.043); see Figure 1.
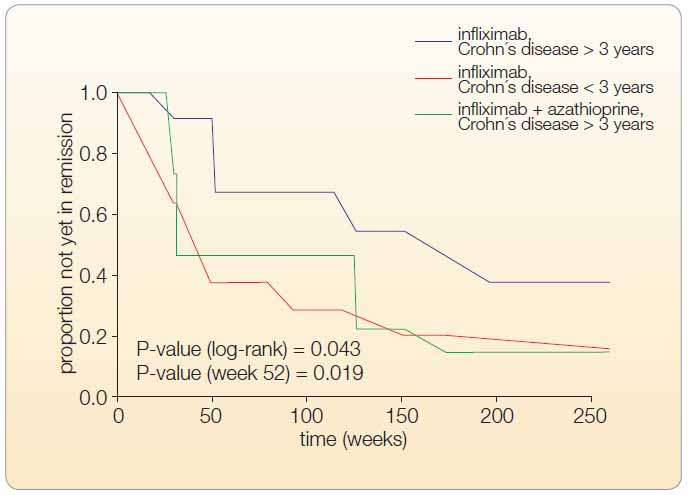 The multivariate analysis showed: 1. GIII vs. GII: GIII had significantly better outcomes than GII; odds ratio (OR) 7.20 95% CI (0.981–52.84; P-value = 0.053). 2. GIII vs. GI: GIII had only marginally better outcomes than GI (P-value = 0.975). 3. GI vs. GII: GI had significantly better outcomes than GII; OR 6.30 95 % CI (0.838–47.373; P-value = 0.052). The proportions of patients who reached partial MH within the first 52 weeks with a SES-CD of < 5.5 were as follows: 10/21 in GI, 8/19 in GII, and 11/20 in GIII. The percentages at 95% CI are given in Table 2.
The multivariate analysis showed: 1. GIII vs. GII: GIII had significantly better outcomes than GII; odds ratio (OR) 7.20 95% CI (0.981–52.84; P-value = 0.053). 2. GIII vs. GI: GIII had only marginally better outcomes than GI (P-value = 0.975). 3. GI vs. GII: GI had significantly better outcomes than GII; OR 6.30 95 % CI (0.838–47.373; P-value = 0.052). The proportions of patients who reached partial MH within the first 52 weeks with a SES-CD of < 5.5 were as follows: 10/21 in GI, 8/19 in GII, and 11/20 in GIII. The percentages at 95% CI are given in Table 2.
The differences in the rates of patients who reached partial MH at week 52 among groups I, II, and III were nonsignificant (in the log-rank test, P = 0.509) as well as the differences in the overall survival (in the log-rank test, P = 0.793).
At week 52, deep clinical remission was observed in seven of 21 patients (33.33%) in GI, in three of 19 patients (15.7%) in GII, and in eight of 20 patients (40%) in GIII. The median CDAI score was 60 (40–80) points and the median CRP level was 3.56 mg/L for all groups. At week 52, partial endoscopic remission was reached in 10 of 21 patients (50%) in GI, in eight of 19 patients (44.8%) in GII, and in 11 of 20 patients (60.9%) in GIII. The median CRP level was 4.08 and the median CDAI score was 95 (40–125).
 Thirteen of the 60 primary responders did not respond to IFX treatment any longer. One patient in GI had an extensive small intestine and colonic disease; seven patients in GII and one patient in GIII underwent surgery for stenosis at weeks 16, 27, 32, 72, 128, and 176, see Figure 2.
Thirteen of the 60 primary responders did not respond to IFX treatment any longer. One patient in GI had an extensive small intestine and colonic disease; seven patients in GII and one patient in GIII underwent surgery for stenosis at weeks 16, 27, 32, 72, 128, and 176, see Figure 2.
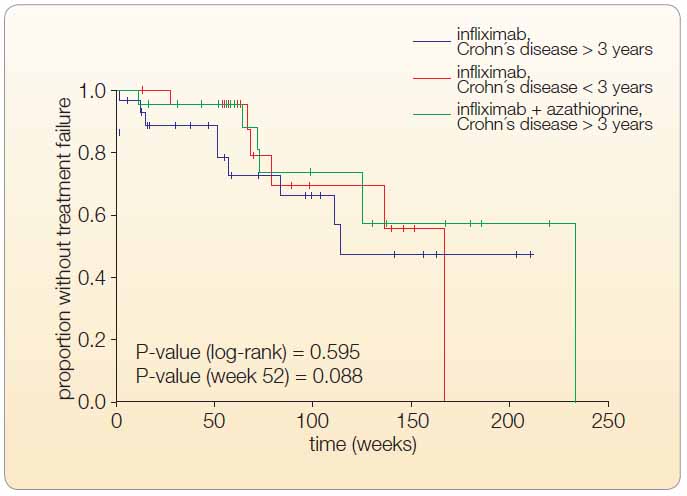 Kaplan-Meier estimates of the rates of patients with decrease in efficacy, see Table 2 and Figure 3. The differences among groups were significant only until the end of week 52 (P = 0.039), but not overly (P = 0.116 in the log- -rank test). Four of 60 patients had antibodies to IFX (ATI) between weeks 30–52 (one in GI, two in GII, and one in GIII). In 10 of 47 patients, it was necessary to reduce the interval between infusions or to increase the IFX dose (in two patients in GI, six in GII, and two in GIII), see Figure 3.
Kaplan-Meier estimates of the rates of patients with decrease in efficacy, see Table 2 and Figure 3. The differences among groups were significant only until the end of week 52 (P = 0.039), but not overly (P = 0.116 in the log- -rank test). Four of 60 patients had antibodies to IFX (ATI) between weeks 30–52 (one in GI, two in GII, and one in GIII). In 10 of 47 patients, it was necessary to reduce the interval between infusions or to increase the IFX dose (in two patients in GI, six in GII, and two in GIII), see Figure 3.
Steroid tampering during the IFX treatment: Corticosteroids were given to 45.5% of patients in GI, 41.37 % in GII, and 26.1% in GIII. Corticosteroid treatment was discontinued in GI after a median of 6.8 weeks (IQR 10.2– 18.0), in GII after a median of 29.0 weeks (IQR 16.9–35.5), and in GIII after a median of 13.2 weeks (IQR 12.1–14.3). Concomitant therapy with corticosteroids had no influence on the MH rates (P = 0.14).Infusion reactions that led to discontinuation of treatment occurred in 12% of patients, 5.4% of them had delayed infusion reaction at week 30, 4.05 % of patients developed serum sickness-like reaction (three consecutive cases observed at weeks 52, 136, and 152). A 22-year-old woman developed parapsoriasis at week 52.
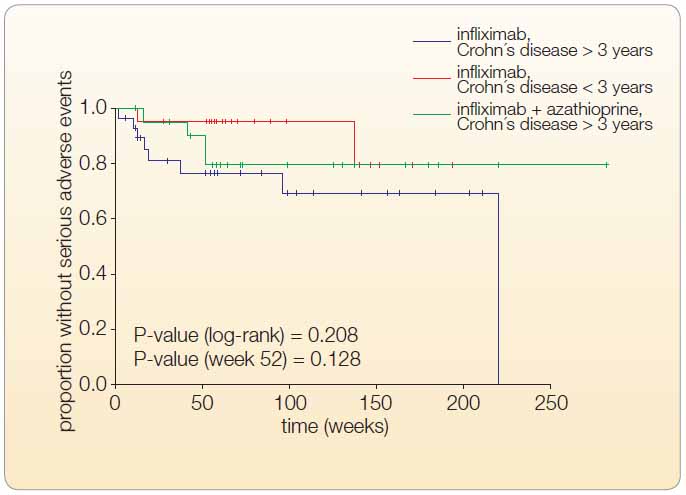 SAEs including one case of herpes zoster were diagnosed in 6.75% of patients. A 60-year-old woman receiving IFX + AZA combination therapy developed tuberculosis approximately three months after a negative Quantiferon test and a negative chest radiograph. Three patients stopped IFX therapy because of neurological complications (a 32-year-old man developed serous meningitis after the second infusion, a 31-year-old man died from fatal sepsis at week 152, and a 55-year-old woman was diagnosed with a central demyelinating lesion at week 30), see Figure 4.
SAEs including one case of herpes zoster were diagnosed in 6.75% of patients. A 60-year-old woman receiving IFX + AZA combination therapy developed tuberculosis approximately three months after a negative Quantiferon test and a negative chest radiograph. Three patients stopped IFX therapy because of neurological complications (a 32-year-old man developed serous meningitis after the second infusion, a 31-year-old man died from fatal sepsis at week 152, and a 55-year-old woman was diagnosed with a central demyelinating lesion at week 30), see Figure 4.
Discontinuation of IFX treatment: Twenty-three percent of patients discontinued IFX therapy; 4.03% ofpatients were primary non-responders, one patient was non-compliant, 12% of patients developed severe infusion reaction, and 6.75% had SAEs.
13.5% of patients were switched to adalimumab.
Mortality during IFX therapy was 2.70% (2 of 74 patients died), but was unrelated to IFX treatment.
Conclusion: One third of patients in both GIII (CD > 3 years, IFX + AZA) and GI (CD < 3 years, IFX) and only every tenth patient in GII (CD > 3 years, IFX) reached deep clinical remission at week 52. Timely infliximab monotherapy works best in patients with evidence of active inflammation as demonstrated by an elevated C-reactive protein level and in nonstricturing disease. Multiple small bowel strictureplasties during IFX treatment were without serious complications.
Discussion
In our study, we evaluated the outcomes of the biological treatment with IFX in patients with moderate to severe Crohn’s disease during period from March 2003 to December 2010.
Mucosal healing was achieved at the end of week 52 of treatment in 63.5% of patients, but complete mucosal healing was observed in 30% of patients only. The greatest benefit from treatment was seen in the patients with complete MH. Those with partial MH with a SES-CD of < 5.5 were in clinical remission.
The greatest benefit from IFX was seen in patients in GIII (treated with IFX and AZA), and in patients in GI (with short duration of the disease; median of 0.8 years; IQR 0.5–1.3) who were early treated with IFX only after several weeks of corticosteroid therapy.
We were interested in IFX treatment outcomes in patients who underwent prior long-term, conventional therapy with steroid pulses and immunosuppressive drugs. The long duration of the disease (median of 9 years) with advanced changes in the intestine were the reasons behind the small number of patients in GII reaching complete endoscopic remission at the end of week 52.
These patients were around 30 years old with extensive form of CD and a CDAI score of > 220 points. All patients had diffuse inflammatory and fibrotic changes in the intestine. Patients with intestinal strictures due to CD may have less response to the treatment and are at risk of developing acute bowel obstruction [9]. This group had the largest number of SAEs (21.01% at week 52). Abdominal operations due to the stricturing CD were performed in seven of 19 patients.
The duration of the disease, abdominal surgery, and corticosteroid and immunosuppressive therapy before the first IFX infusion did not influence outcomes of treatment with IFX. CD severity and duration are the only independent predictors of stenosis [9].
Conventional therapy is aimed on the control of inflammation but does not change the natural course of the disease [10]. The current standard of medical practice consists of a sequential (step-up) approach starting with the mildest form of medication and moving to next step (anti-TNF-alfa agents) in non responders. This method of treatment is not very effective for inducing musocal healing and for preventing disease progression in patients with bad prognosis [10–12]. Repeated pulses of corticosteroids and immunosuppressive agents significantly increase the risk of side effects. Optimization of a conventional treatment with corticosteroids and immunosuppressants in inflammatory bowel disease was established under the Education Program EHEAD at the international symposium in Brussels in September 2010 [13].
Tumor necrosis factor antagonists induce and maintain mucosal healing and significantly reduce surgery and hospitalization rate. MH is the sole outcome predictor. Complete MH in patients with early stage CD is associated with significantly higher steroid-free remission rates 4 years after the therapy began [14].
Early use of biologic drugs may improve patients’ outcomes with active CD. Early biological treatment should be indicated only in patients with poor prognosis (significant perianal disease, duodenal involvement, deep ulcers, young age at diagnosis (below 40 years) and in patients who need treatment with corticosteroids from the begining of disease) [15]. However, accurate prognostic markers must be identified to guide patients’ selection.
TNF-alfa antagonists may be more effective in patients with disease duration less than 1 or 2 years. Ideally duration of less than 1 year should be a part of the definition of early Crohn’s disease [10, 16].
Early Crohn’s disease is characterized by clinical, endoscopic and radiologic signs of active inflammation without fistula, abscess and stricture [10].
The SONIC trial assessed the efficacy of IFX, AZA or combined therapy with IFX and AZA in patients with moderate to severe Crohn´s disease, who had not been treated previously any biological agents or immunosuppressants. The duration of disease was 2.2 years, which is one of the shortest periods. 96 of 169 patients receiving combination therapy (56.8%) were in corticoid-free remission at week 26 as compared with 75 of patients (44.4%) receiving IFX alone (P = 0.02) and 51 of 170 patients (30%) receiving AZA alone. Similar trends were found at week 50. Serious adverse events were similar among groups: 3.9% of pts in the combination therapy group, 4.9% in the IFX group and 5.6% of those in the AZA group. Infusion reactions occurred less frequently among patients receiving combination therapy [17].
In the cohort study, published in 2009, Schnitzler has analyzed scheduled and episodic IFX treatment in 547 single centre patients with CD during 4.6 years or 55 months of follow-up (IQR 27–83). Sustained benefit has been observed in 347/547 patients (63.4%) receiving long- -term treatment. Patients who achieved complete or partial MH at follow-up endoscopy had lower rates of major abdominal surgeries (14.1 %) compared to patients who did not exhibit MH (38.4%), P < 0.001 [18].
IFX therapy combined with an immunosuppressant is the best strategy to achieve optimal results in patients at high risk of disabling disease. The high-risk patients are under 40 years of age at the time of diagnosis, need corticosteroid therapy from the beginning of the disease, have perianal disease, and undergo surgery within 5 years of diagnosis. If two of the three criteria were present at diagnosis, then 84% of patients had disabling disease in five years and if all three risk factors were present, the respective figures were 91% and 93% [19].
The IFX + AZA combination therapy is highly effective, but in clinical practice, it should be applied very judiciously. Clinicians need to remain aware of the SAEs during long-term therapy beyond the confines of clinical trials [20–22].Conclusion
The authors would like to contribute by this work to the long-debated question of bilogical therapy optimization in patients with Crohn’s disease.
Long-term biological treatment of Crohn’s disease is clearly beneficial to patients. IFX therapy is well tolerated and safe, and at least 63.5% of patients have a long-term benefit from it.
Seznam použité literatury
- [1] Cosnes J, Cattan S, Balin A, et al. Long-term evolution of disease behaviour of Crohn’s disease. Inflamm Bowel Dis 2002; 8: 244–250.
- [2] Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: The ACCENT I randomised trial. Lancet 2002; 359: 1541–1549.
- [3] Lichtenstein GR, Hanauer SB, Sandorn WJ. Management of Crohn’s disease in adults. Am J Gastroenterol 2009; 104: 465–483.
- [4] Sandborn WJ, Feagan BG, Hanauer SB, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology 2002; 122: 512–530.
- [5] Daperno R, Sostegni A, Lavagna L, et al. The role of endoscopy in inflammatory bowel disease. Eur Rev Med Pharmacol Sci 2004; 8: 209–214.
- [6] D’Haens G. Endoscopic and Histological Grading in IBD. Inflammatory Bowel Diseases 2008, 3rd Congress of ECCO – The European Crohn´s and Colitis Organization. Cité – Centre de Congrés Lyon, France, February 28 – March 1, 2008.
- [7] Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new simplified endoscopic activity score for Crohn´s disease: the SES-CD. Gastrointest Endosc 2004; 60: 505–512.
- [8] Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biometrical Journal 2008; 50: 346–363.
- [9] Lichtenstein GR, Olson A, Travers S, et al. factors associated with the development of intestinal strictures or obstructions in patients with Crohn’s disease. Am J Gastroenterol 2006; 101: 1030–1038.
- [10] Peyrin-Biroulet L. Early Crohn´s Disease: a proposed definition for use in disease-modification trials. Gut 2010; 59: 141–147.
- [11] D’Haens G, Baert F, van Assche G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet 2008; 371: 660–667. References
- [12] Ordás I, Feagan BG, Sandborn WJ. Early use of immunosuppressives or TNF antagonists for the treatment of Crohn’s disease: time for a change. Gut 2011; 60: 1754–1763.
- [13] Panaccione R, Hibi T, Peyrin-Biroulet L, Schreiber S. Implementing changes in clinical practice to improve the management of Crohn´Disease. J Crohns Colitis 2012; 6 Suppl 2: S235–S242.
- [14] Baert F, Moortgat L, D´Haens G, et al. Mucosal Healing Predicts Sustained Clinical Remission in Patients With Early-Stage Crohn’s disease. Gastroenterology 2010; 138: 463–468.
- [15] Beaugerie L, Seksik P, Nion-Lamurier L, et al. Predictors of Crohn’s disease. Gastroenterology 2006; 130 (3): 650–656.
- [16] Schreiber S, Reinisch W, Colombel JF, et al. Early Crohn´s Disease shows high levels of remission to therapy with adalimumab: sub- -analysis of CHARM. Gastroenterology 2007; 132: 147 A.
- [17] Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, Azathioprine, or Combination Therapy for Crohn’s disease. N Engl J Med 2010; 362:15, 1383–1395.
- [18] Schnitzler F, Fidder H, Ferrante M, et al. Long-term outcome of treatment with infliximab in 614 patients with Crohn’s disease. Gut 2009; 58: 492–500.
- [19] Seksik P, Loftus EV, Beaugerie L, et al. Validation of predictors of 5-year disabling CD in a population-based cohort from Olmsted Country, Minnesota 1983–1996. Gastroenterology 2007; 132: A17.
- [20] Colombel JF, Loftus EV, Christensen R, et al. The safety profile of Infliximab in patients with Crohn’s disease. The Mayo Clinic experience in 500 patients. Gastroenterology 2004; 126: 19–31.
- [21] Cottone M, Kohn A, Dapermo M, et al. Advanced age is an independent risk factor for severe infections and mortality in patients given anti-tumor necrosis factor therapy for inflammatory bowel disease. Clin Gastroenterol Hepatol 2011; 9: 30–35.
- [22] Cottone M, Criscuoli V. Infliximab to treat Crohn’s disease: an update. Clin Exp Gastroenterol 2011; 4: 227–238.